
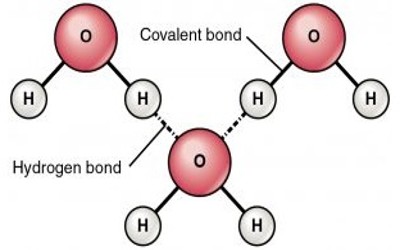
Intermolecular forces are between a compound and another compound, so let's talk about those guys, okay so the 3 main IMFs the first one being called hydrogen bonds this is deceiving because hydrogen bonds are not really bonds, they're not bonds but they're called bonds because they're very strong in comparison to the rest of them. Those 3 are intramolecular forces they're within a compound. Covalent bonds are also intramolecular forces and they're actually the bonds between the sharing of the valence electrons, there's the covalent bond that's actually intramolecular forces.Īnd then lastly the metallic force, it's the metal cations with mobile electrons kind of flowing between the cations the sea of electrons. Intramolecular forces you might be familiar with, ionic bonds are intramolecular forces, how the cations and ions are attracted to each other. It's not, you can't communicate with people everywhere, so there are differences with if intermolecular forces and intramolecular forces.
But if you have intranet off your school or your maybe you have a job has something called intranet that is just within that particular school or within that particular job.
So an easy way like you think, when you think internet like the internet is how you interact with other people in the out of the whole entire world.

Alright so we're going to talk about intermolecular forces, you might also have heard that as IMF That is the attraction between 2 or more molecules, I hope you don't get that confused with intramolecular forces.


 0 kommentar(er)
0 kommentar(er)
